Element’s regulatory advisors have 30 years of domestic and international medical device regulatory consulting experience, specializing in handling the most complex challenges and partnering with you on the path to submission.
Our approach to medical device regulatory consulting
Element offers a unique approach to assist from early stage design through commercialization. Element’s multidisciplinary team of experts has a deep understanding of the medical device product life cycle, and the impact certain decisions can have on regulatory success.
Element offers a talented team with a wide range of expertise that is ready to support your goals. With 16 laboratories in our life sciences division to help support your projects, you can leverage a one-stop-shop for all of your project needs.
The Element advantage
Our advisors have worked with a multitude of clients, including some of the world’s largest medical device manufacturers, and will ensure you are receiving the proper guidance for all stages of product development and commercialization goals. Trusting the right partner for your business is critical and Element’s position in the medical device industry across multiple segments, helps drive the certainty of a successful submission.
Contact us today to schedule a consultation.
More from Element

Medical Device Testing
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.
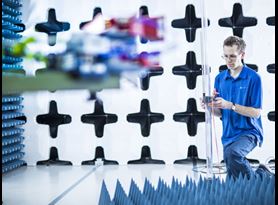
EMI & EMC Testing
Element performs electromagnetic compatibility (EMC) and electromagnetic interference (EMI) testing for a variety of medical devices and components, including implantable devices, diagnostic tools and therapeutic equipment.

Medical Device Battery Testing
Element provides safety and certification testing for rechargeable lithium-ion and nickel metal hydride batteries used in hospital and home health applications.

Orthopedic Implant Testing
As a global leader in orthopedic implant testing, Element has years of experience in evaluating hip replacements, knee prostheses, spinal devices and many other implants.


