Element provides biocompatibility analysis and toxicology services as per ISO 18562 for breathing gas pathway devices. We work closely with customers to help identify all necessary testing and compliance requirements to support global device approval from relevant regulatory bodies.
What is ISO 18562 testing?
The ISO 18562 series of standards outlines the general principles for the testing required for the evaluation of breathing gas pathways of medical devices.
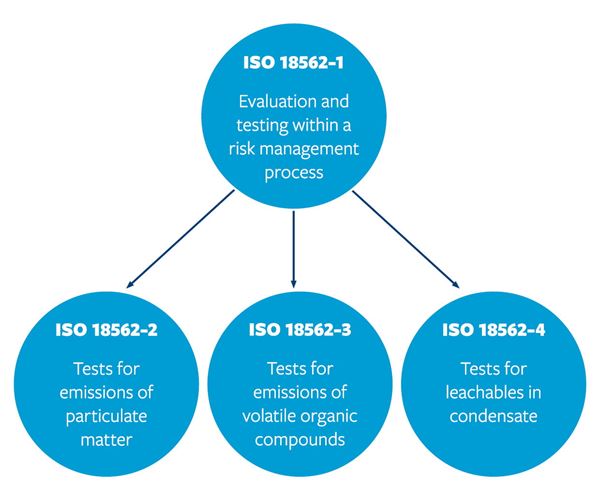
We support with ISO 18562 compliance across four parts, as shown below. Our laboratories utilize state-of-the-art equipment such as thermal desorption gas chromatography mass spectrometry (GC-MS), direct injection GC-MS, Inductively coupled plasma mass spectrometry (ICP-MS). to help you achieve global market access.
The key benefits of ISO 18562 testing
- Regulatory Compliance
- Enhanced Patient Safety
- Risk Management
- Global Market Access
- Quality Assurance
- Comprehensive Analysis
- Technical Expertise and Insights
- Efficient Path to Approval
- Customized Testing Solutions
Enhance your patient safety
Enhanced patient safety of breathing gas pathway devices involves identifying and mitigating potential hazards like sensitization, irritation, toxicity, carcinogenicity, mutagenicity, and genotoxicity, thereby safeguarding patient health. This is achieved through a comprehensive risk management framework that offers a structured approach to evaluating and managing risks associated with these devices, ultimately enhancing their safety and reliability.
Additionally, quality assurance measures play a vital role by ensuring the integrity and quality of these medical devices through rigorous testing methodologies and adherence to Good Laboratory Practice (GLP) quality systems, further bolstering patient safety.
Utilize our technical expertise
Our technical expertise in ISO 18562 testing involves the utilization of industry-leading equipment and highly skilled personnel to conduct thorough analyses. We strictly adhere to ISO 18562 standards to ensure that you have absolute trust in the reliability of our analysis.
Our comprehensive analysis covers a wide range of device biocompatibility aspects, such as particulate matter emissions, volatile organic compounds (VOCs), and leachables in condensate, allowing for a holistic evaluation of product safety. We offer customized solutions through close collaborating to understand your specific testing and compliance needs, tailoring our approach to ensure regulatory requirements are met effectively. Additionally, we provide transparent documentation of testing procedures and results to enable stakeholders to evaluate the biocompatibility of the tested product.
We support your regulatory compliance journey
Element supports your regulatory approval journey through ISO 18562 testing, leveraging a worldwide network of medical device testing laboratories specializing in assessing breathing gas pathways for respiratory and ventilation devices and accessories. Our expertise ensures that your medical devices meet the regulatory standards set by organizations like the U.S. FDA and EU MDR, thereby expediting the approval process and minimizing delays to market.
We provide guidance and assistance in navigating the regulatory landscape, helping companies streamline approval procedures. Our comprehensive ISO 18562 studies assess hazards associated with breathing gas pathway devices, including leachable condensates, particulate matter, and Volatile Organic Compounds (VOCs), ensuring product biocompatibility for medical use and facilitating global market access.
Why choose Element?
With one of the largest and most experienced Extractables and Leachables testing practices in the world, in addition to unparalleled experience testing a breadth of products that extend from pharmaceuticals, biologics, and devices, to consumer products and more, as your regulatory and scientific partner, Element will help you to navigate the most efficient path to regulatory approval.
Element Materials Technology Analytical Testing Services conducts all studies with the highest degree of scientific integrity, under our Quality Management System. Our analytical laboratories feature the latest state-of-the-art equipment that we operate in compliance with Good Laboratory Practice (GLP) quality systems.

Watch our ISO 18562 Webinar
Watch our webinar for an in-depth overview of ISO 18562 testing, focusing on the bio-compatibility evaluation of breathing gas pathway devices. Nick Morley delves into the analytical methodologies essential for supporting this evaluation as per ISO 18562.
Failure to comply can result in costly recalls, potential harm to patients, and damage to a company's reputation and revenue. The webinar addresses these concerns by detailing the testing procedures mandated by ISO 18562 and highlighting real-world examples where inadequate testing led to significant repercussions.
Watch TodayDownload our ISO 18562 biocompatibility evaluation white paper for free
Learn more about the importance of biocompatibility studies in the R&D stages of medical product development.
We delve into the four parts of ISO 18562 and how effective studies help support product compliance and reduce delays to market.
This white paper was authored by Mike Ludlow Market Development Manager for Extractables & Leachables for Life Sciences Manchester.
downloadRead our blog post in response to the FDA class 1 recall of 2 million medical devices
Demand for ISO 18562 medical device testing has accelerated as the result of class 1 medical device recall (MDR).
The recall involves various types of mechanical ventilators, including bi-level airway pressure (BiPAP) and, continuous positive airway pressure (CPAP).
Welcome to Element Manchester
Our Manchester laboratory is home to over 50 life science experts dedicated to helping our clients ensure patient safety.
We bring cutting-edge extractable & leachable techniques, state-of-the-art equipment, and knowledge of regulatory standards to provide high quality analytical data to help you make key decisions on your product.
Your global partner from discovery to market
Still researching? Here are a handful of our many services to help achieve regulatory approval quickly and efficiently.

Extractables and Leachables Studies
Our extractables and leachables studies offer tailored solutions that ensure patient safety and compliance with industry standards.

Pharmaceutical and Biopharmaceutical Unknown Identification and Impurity Testing
Our experts work closely with customers at every stage of the product lifecycle to identify unwanted compounds and ensure products are pure, safe, and quality.
Troubleshooting and Updating Analytical Methods
Element provides troubleshooting and updating analytical methods services to help you achieve more consistent product quality, more efficient manufacturing, reduce cost and save money.

Excipient Raw Materials and Container Testing
Element has comprehensive capabilities that can be applied to the testing of excipient, raw materials, and pharmaceutical containers, enabling you to ensure the quality and safety of your product.

Forced Degradation Studies
Element’s forced degradation, stress testing services per ICH Q1A guidelines assess the stability of drug substances or drug products with effects on purity, potency, and patient safety.

Pharmaceutical Stability Storage and Testing
Element offers advanced pharmaceutical stability testing and ICH storage services to ensure the maintenance of product quality, safety and efficiency throughout the shelf life.

Elemental Impurity Testing and Analysis
Element’s trace metal laboratories provide expert elemental impurities testing and analysis in compliance with USP and ICH Q3D guidelines to ensure the safety of drugs and drug products.

CMC Product Development Services
Our CMC product development services include formulation development, parenteral and topical product development, microbiology testing services, and consultancy.