Topical Drug Delivery Systems: An Overview
In this article, Element's experts reveal how topical drug delivery systems bypass first-pass metabolism while overcoming skin barrier challenges to achieve therapeutic effects.
This comprehensive guide explores percutaneous absorption pathways, Fick's Law applications, and proven enhancement methods - from formulation optimization to physical techniques - helping drug developers navigate the complexities of creating effective topical pharmaceuticals.
Topical drug delivery systems can, at a very high level, be classified as having either external or internal applications.
External topical dosage forms are applied to the affected area of the cutaneous (skin) surface, while internal topical dosage forms are applied to a mucous membrane, such as the eye (conjunctiva), ear, nasal cavity, oropharyngeal cavity, vagina, or anorectal region/tissue for local activity. Topical dosage forms are then classified further according to their physical state, i.e., solid, liquid, and semisolid, as detailed in Figure 1. While topical medications are intended to have a local effect, they may also cause systemic effects.
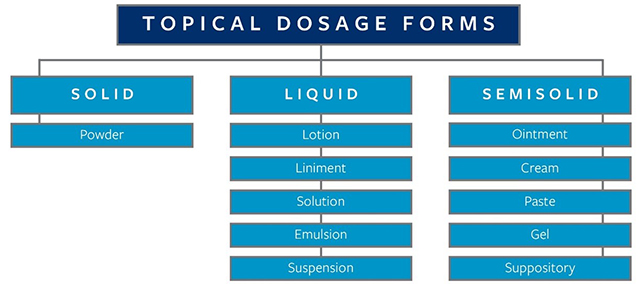
Figure 1. High-level overview of topical dosage forms
The benefits and challenges of topical formulations
While topical dosage forms offer a wide range of advantages, topical drug delivery systems also present challenges and present their own unique set of disadvantages and limitations.
Advantages of topical drug products
One of the primary benefits of topical drug delivery systems is the avoidance of first-pass metabolism, also known as first pass effect, or pre-systemic metabolism. Particularly for orally administered drugs, which are subject to first-pass metabolism, the drug is metabolized at a specific location within the body, and the concentration of the drug is greatly reduced before it reaches systemic circulation. Topical formulations, which deliver the drug via the skin to achieve systemic therapeutic effect, avoid the challenges associated with first-pass metabolism, as systemic circulation is achieved without being impacted by the phenomenon of the first pass effect. Percutaneous absorption is illustrated in Figure 2.
Moreover, topical drugs can deliver a drug substance more selectively to a specific site, and efficacy can be achieved with a lower total daily dosage of an active pharmaceutical ingredient (API) by means of continuous drug input. Drugs which have a short biological half-life and/or narrow therapeutic window (the concentration range of the drug in plasma where it is efficacious without causing toxic effects in most people; also known as therapeutic range) are ideal candidates for topical delivery systems.
Quite often, the convenience, ease of application – including their suitability for self-administration and medication, improved adherence and compliance, and termination of use of topical drug products are cited as key advantages and benefits. When compared to enteral or parenteral routes of administration, topical drugs present less risk and inconvenience. Topical routes of administration are not influenced by various conditions that can impact the absorption of parenterally or enterally administered drugs, such as fluctuations in pH, gastric emptying time, and gastro-intestinal incompatibility. While fluctuations in drug levels are seen with other routes of administration, this is avoided with topical drug delivery systems, as has also been observed with inter- and intra-patient variations.
Disadvantages and limitations of topical drug delivery systems
Although skin can be an ideal site to achieve both local and systemic effects via the delivery of drug substances, it also acts as a barrier, preventing the penetration of many APIs, as drugs which have a larger particle size may not be easily absorbed through the skin. To facilitate skin absorption, the molecular weight (MW) of a compound should be under 500 Dalton when developing drug substances for use in topical dermatological or percutaneous systemic therapies. Furthermore, for optimal permeability, lipid/ water partition coefficient is expected to be 1 or greater. Therefore, it is particularly challenging for drugs with a larger particle size to absorb through the subcutaneous layer. It is also important to note that some drugs have poor permeability through skin.
Drug developers are also faced with challenges around enzymes contained in the epidermis layer, as some may denature drugs, which can lead to variations of molecular structure and the alteration of topical bioavailability. Skin irritation and contact dermatitis, as well as allergic reactions, may occur in some patients, which can be a result of contact with excipients or the active pharmaceutical ingredient. Perhaps the greatest limitation for topical drug delivery systems is topical formulations are only appropriate for drugs which require limited plasma concentration to achieve the desired therapeutic effect and action.
Absorption of drug substances through skin
A multi-layered organ, the skin serves as a barrier, and is comprised of three layers, the epidermis, dermis, and hypodermis. Dermal absorption refers to the ability of a substance to permeate the skin and enter the body. Dermal absorption encompasses penetration (the entrance of a substance into the skin layer), permeation (penetration of the substance from one layer the next), and resorption (the uptake of the substance, most often leading to systemic circulation).
There are two primary routes of absorption through skin, trans epidermal absorption, and trans follicular (shunt pathway) absorption, as detailed in Figure 2. Trans epidermal absorption is primarily responsible for diffusion across the skin, and any resistance along this pathway most often is observed in the stratum corneum. In the trans epidermal pathway, permeation involves partitioning of the drug into the stratum corneum. The trans follicular (shunt pathway) relies upon the skin’s appendages, primarily sebaceous glands, sweat glands, and hair follicles, as secondary avenues for permeation, which are all considered to be shunts bypassing the trans-epidermal route. Due to the relatively large opening of follicular pores, the follicular route is particularly important when considering permeation. Partitioning of the drug takes place into the sebum, followed by the diffusion into the depths of the epidermis when the shunt pathway is utilized for absorption.
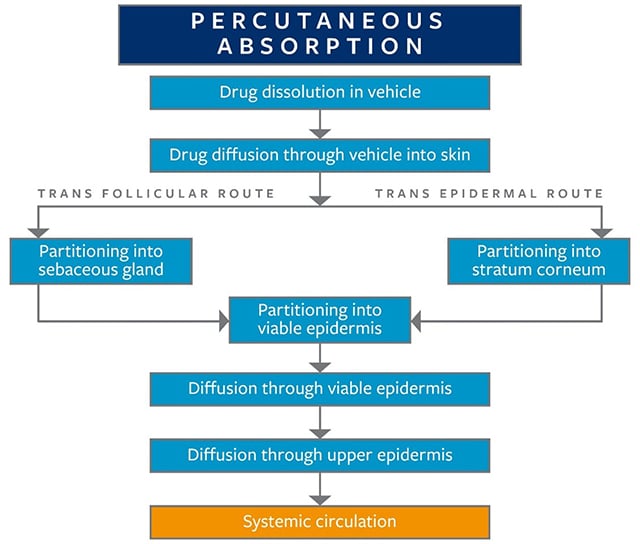
Figure 2. Dermal (percutaneous) absorption of active pharmaceutical ingredients (APIs) or chemicals from the outer surface of the skin to systemic circulation
Factors impacting drug permeation through skin
The rate of transport of a drug across the stratum corneum follows Flick’s Law of Diffusion, which states the rate of diffusion of a substance across the unit area (i.e., surface or membrane) is proportional to the concentration gradient. There are several factors that impact the extent and rate of topical and percutaneous drug absorption and transportation, including the physiology and pathology of the skin, such as hydration, blood flow, and lipid content. It is important to note that percutaneous absorption is decreased in instances where patients have an increased thickness of the stratum corneum, while increased absorption is observed when topical drug products are applied to traumatized and/or inflamed skin.
Physicochemical properties of drugs and excipients also have an impact on the permeation of topical drug formulations. Consideration needs to be given to the partition coefficient, pH, drug solubility, concentration of the drug substance in the formulation, particle size of the API, and molecular weight. Additionally, polymorphism can compromise the quality and bioavailability of a drug product due to changes in physicochemical properties, specifically solubility.
Furthermore, the composition of the delivery system itself needs to be evaluated, and consideration given to release characteristics and the nature of the vehicle used in the formulation. The vehicle facilitates drug delivery by way of bringing the drug substance into contact with the skin. The vehicle used within a topical formulation should not interact with or deactivate the drug substance, and should be stable, non-irritating, non-allergenic, and facilitate ease of use. Most vehicles are created from a blend of one of three primary components, aqueous solvents, powder, and oil, with thickening and emulsifying agents, buffers, antioxidants, preservatives, propellants, etc. The vehicle itself may provide benefits, as occlusive vehicles enhance penetration and improve efficacy. Cooling, drying, emollient, or protective qualities may also be attributed to the vehicle chosen for a topical formulation. However, the vehicle can produce unwanted side effects if it is found to be excessively drying or occlusive.
Other factors that increase percutaneous absorption include drug substances with lower molecular weight, higher concentrations of drug substances, vigorous rubbing at the time of application, and the use of lipophilic compounds. There are several methods available to drug developers that can be used to improve drug permeation, which fall into three primary categories: formulation optimization, physical enhancement, and chemical enhancement, as outlined in Figure 3, which contains examples of each.
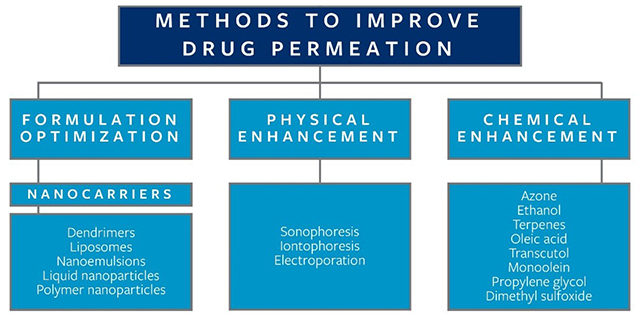
Figure 3. Methods to improve topical drug permeation through skin, including examples
CDMO partnerships support topical drug development
There are several factors to consider when evaluating a contract research and development organization (CDMO) to support topical drug development. While capacity, technical expertise, experience, and facilities are key aspects that need to be contemplated, communication, collaboration and partnership play an important role in the drug developer/CDMO relationship. Without a true partnership, clear and open communication, and effective collaboration, the challenges inherent to topical drug development will be even more difficult to overcome.
As a full-service CDMO, Element provides comprehensive, end-to-end support for the entire drug development life cycle, from discovery to small-scale manufacturing. We are true collaborators and innovators, and our teams are fully invested in helping our customers achieve their goals. Dedicated project managers facilitate open and frequent communication, and our customers have direct access to the formulators and scientists who are advancing the development and analysis of their products. With a proven track record of success in developing a wide range of topical dosage forms, including those with challenging and complex APIs, our team stands ready to partner with you - connect with us today.
Related Services

Chemistry, Manufacturing and Controls (CMC) & CDMO Testing Services
Element provides specialized analytical testing services designed for CDMOs, offering flexible capacity, regulatory compliance, and advanced capabilities that enable successful client program delivery across all development phases.


