Element offers personalized, global-scale Life Sciences testing addressing complex regulatory challenges. Our accredited laboratories specialize in Pharmaceutical, Medical Devices, Biotech, Antimicrobial, Food, and Environmental testing. We support laboratories as a premier supplier of laboratory consumables, training, consultancy, automated solutions, and technical services. With over 40 years of experience, we provide certainty in results, risk mitigation, and dedicated support from R&D to manufacturing.
Key Benefits of Life Sciences Testing Services
- Global Expertise, Personalized Solutions: Receive expert, personalized testing on a global scale throughout your Life Sciences product development, tailored to your specific needs, offering you support from start to finish.
- Accredited State-of-the-Art Laboratories: Benefit from cutting-edge ISO/IEC 17025 accredited labs and highly qualified teams, ensuring accurate and reliable testing for a wide range of products, from pharmaceuticals to environmental samples.
- Flexible Workflow Capacity to Meet Your Needs: Add workflow capacity or access on-demand specialist testing, including full-scale studies for pharmaceutical, medical, or consumer products, offering flexibility from laboratory to location-based testing to fit your project.
- Integrated Lab Solutions: Enhance your lab operations with Element Lab Solutions, offering curated instruments, automation, and consumables, along with expert consultation for method development, implementation, training, and ongoing support.
- Trusted Regulatory Compliance Partner: Partner with a leading Testing, Inspection, and Certification organization, ensuring regulatory compliance at every stage of your Life Sciences product development. Rely on our unrivaled history and experience, helping you to navigate the most complex challenges.
Element in Life Sciences
Dedicated to excellence, Element’s accredited laboratories adhere to strict industry testing standards and specifications. Our commitment to quality is emphasized through our credentials in ISO/IEC 17025, GLP (Good Laboratory Practice), BSL-3, and BSL-2 (Biosafety Level 2 & 3). We provide peace of mind through tailored solutions, expertise, state-of-the-art equipment, guidance, and transparent communication.
For more information about our Life Sciences services and solutions, to request a quote, or discover our team of experts, contact us today.
Find out more about Element and our credentials within the testing, inspection, and certification industry.
Life Sciences Testing Services
Element offers a diverse, comprehensive suite of customized testing solutions and products for clients in the Antimicrobial, Food Production, Medical Device and Pharmaceutical industries.

Analytical Chemistry Testing
We offer our clients full product and analytical chemistry solutions. Our GLP and cGMP chemistry offerings include chemical characterization of active ingredients, testing of product-specific impurities, physical chemistry analysis and storage stability testing.

Pharmaceutical Testing
Element’s pharmaceutical laboratories provide specialist pharmaceutical testing services, including chemical, physical, microbial, and stability testing on a vast array of products, from raw materials to finished products.

Medical Device Testing
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.

Nicotine, Tobacco, ENDS & ANDS Testing
Element's experts provide efficient and reliable nicotine, tobacco and e-liquid testing of ENDS and ANDS products to help you confidently navigate FDA PMTA.
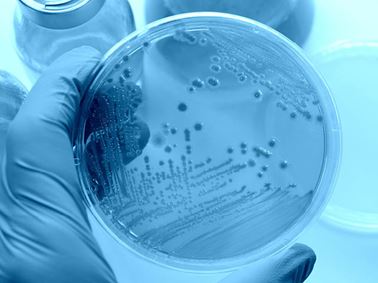
Antimicrobial Testing
With more than 30 years of experience, we are the premier contract antimicrobial testing laboratory and expert partner to the developers, manufacturers and users of antimicrobial pesticide and biocide products.

Food Testing
Element offers a comprehensive food testing package to help you deliver safe and quality food products to market quickly.

Environmental Testing
Element helps its customers to meet their environmental obligations through our comprehensive range of laboratory and field based environmental testing services.

Chromatography Instrumentation & Consumables
Element provide a wide selection of chromatography columns, consumables, and instrument parts from leading manufacturers. From HPLC or GC columns and vials, to sample preparation products and gas generators.

Life Sciences Insights & Resources
Explore cutting-edge insights on testing in our diverse collection of resources:
- Articles
- White papers
- Webinars
- Technical bulletins
- Case Studies
Delve into the expertise of Element's industry leaders, spanning across Pharmaceutical, Medical Devices, Biotech, Antimicrobial, Food, and Environmental sectors.
READ OUR LIFE SCIENCES INSIGHTSYour Global Partner Of Choice
Element is one of the leading Testing, Inspection, and Certification (TIC) suppliers, with laboratories and experts across the globe, we’re the partner of choice for OEMs and manufacturers to maximize performance and minimize risk with their products.
Laboratories located in 30 countries within the Americas, Europe, Middle East, Africa, Asia, and Australia
Employees and Engaged Experts worldwide, ready to help you meet your testing requirements
Accreditations and approvals. An independent endorsement of the quality of service we deliver
Customers served worldwide in a diverse range of market-leading scientific, technical, and engineering industries
