The Importance of Particulate Identification in Biologic Formulations
In many cases, one of the causes of unwanted immunogenic responses to biologic therapeutics is thought to be due to large quantities of aggregates and particulates present in biologic formulations.
Aggregate and particulate formation is a critical quality concern, and due to the nature of biologic drugs, aggregates and particulates can form as a result of degradation during formulation, production, handling and/or storage. Additionally, multiple triggers for protein aggregation have been identified, including mechanical stress, impurities, oxidation, shifts in pH and temperature changes. Particulates and aggregates in biologic formulations can present challenges for assay and identification as they can vary significantly in physical size, resulting in the need for multiple analytical methods or techniques to be employed. Furthermore, according to FDA Recalls, Market Withdrawals, & Safety Alerts database, 23 of 59 (39%) recalls, market withdrawals, and safety alerts between 2018 and 2020 resulted from particulate contamination.
Sources and Types of Particulates in Biologics
Particulate matter in biologic formulations is generally classified as extrinsic, intrinsic or inherent. Inherent, extrinsic and intrinsic particulates are defined below. It is important to note that the focus of most impurity analyses and control of particulates is on extrinsic and intrinsic particulates, rather than inherent particulates.
Inherent Particulates
Protein therapeutics naturally contain inherent particulates. Inherent particulates are typically considered to be a generally accepted characteristic of the biologic formulation and are expected to have been fully characterized during drug development and described in regulatory filings and product applications. Inherent particulates may include specific excipients such as human serum albumin, mannitol crystallization, and proteinaceous aggregates in therapeutic proteins. Although inherent particulates are expected and typically accepted, if quantities of inherent particulates are found to be outside of expected or acceptable levels, an investigation should be conducted as if a particle defect was identified.
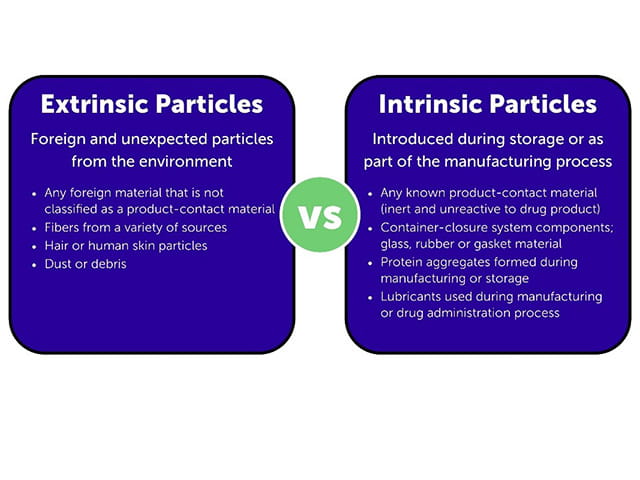
Extrinsic Particulates
Extrinsic particulates are not part of the formulation, assembly or manufacturing process, or container-closure or packaging system. Instead, extrinsic particulates are foreign and unexpected when found in a biologic formulation. Metal, plastic and rubber particles may be identified as extrinsic particulates if the specific substance cannot be tied to any known product-contact material. Because the bioburden of these particulates is uncontrolled and unknown, they pose a greater risk to product sterility, particularly for biologic products that have undergone aseptic processing and packaging.
Intrinsic Particulates
Intrinsic particulates are known to be inert and unreactive to the biologic formulation. Intrinsic particulates result from the container-closure or packaging system, assembly or manufacturing process, or the formulation itself. Sources of intrinsic particulates are traced back to known product-contact material, and may include glass, stainless steel, rubber, or gasket material. When particulates are formed as result of changes in the product over time or due to chemical or physical reactions between components and the product, they are also considered to be intrinsic, as these particulates are resulting from an unexpected reaction and compromised product stability. Examples of such scenarios include oxidation and/or incompatibility of the carrier solution and admixture drug.
Particle Size Matters in Biologic Formulations
Particulates in biologic formulations can come in a variety of shapes, sizes and materials. In addition to the sources and types previously detailed, these particulates can be categorized at a high level as either visible or subvisible.
Visible Particles
Particulates are classified as “visible” when they measure greater than 100-150 microns in diameter. Visible particles can be detected during visual inspection under controlled conditions, whether manual or automated. Pharmacopeial methods provide guidance for the identification of visible particulates, and USP <1> states that “each final container of all parenteral preparations shall be inspected to the extent possible for the presence of observable foreign and particulate matter (“visible particulates”) in its contents. The inspection process shall be designed and qualified to ensure that every lot of all parenteral preparations is essentially free from visible particulates.” It is important to note that transparent particulates may pose challenges for visual inspection and identification, even if they are 100-150 microns in diameter.
Subvisible Particles
Subvisible particles range in size from submicron up to the visible threshold (100 microns). Subvisible particulate limits have been harmonized in the USP, EP and JP and are 6,000 and 600 per container, respectively, for containers ≤ 100 mL. Subvisible particulate control in biologics is critical, as subvisible particles are likely to be injected, posing a greater risk to patients with the potential to affect the drug product’s clinical performance.
Particulates Present Specific Challenges for Cell Therapy Products
While many biologic products undergo final sterile filtration steps, cell therapy products cannot be filtered or purified using such standard approaches during manufacturing. The presence of particulates in cell therapy products presents both a quality and safety concern, and a comprehensive understanding and characterization of particulates in cell therapy products is key. Specific to cell therapy products, particulates have the potential to promote aggregate formation, as they can provide a surface for cellular adhesion. The mechanism of action and efficacy can be negatively affected by cellular aggregates; they can also clog delivery systems.
Drug developers and producers of cell therapy products bear the responsibility of fully characterizing and understanding the formulation and finished product, including the source, composition, potential impact of particulates present and particulate load. Quantitation and identification of particulates in cell therapy products is typically part of the manufacturing release process, and although visual inspection is the most common, a number of methods may be employed. Destructive methods are typically performed as part of process and product validation, as they cannot be applied to each final product. While particulates are likely unavoidable in cell therapy products due to the nature of the product and manufacturing process, understanding the composition and load of particulates in the final product is critical to ensuring the safety and efficacy of the product, as well as ensuring patient safety.
Case Study: Identification of Inherent Particulates in Gene Therapy Drug Products
Challenge: A gene therapy manufacturer requested help in identifying unknown particulates that were observed in their drug product vials during routine quality control testing.
Approach: We developed a step-wise approach to identify the particulates using a variety of techniques, including FT-IR and SDS-PAGE analysis.
Result: Our step-wise approach successfully identified the particulates in the client’s drug product vials as being inherent particulates that were composed of aggregated drug product.
Case Study: Identification of Inherent and Intrinsic Particulates in Cell Therapy Drug Products
Challenge: A cell-based immunotherapy client requested help in identifying unknown particulates observed in their drug product vials during their quality control process. The client requested that the unknown particulates be isolated prior to identification, due to the presence of cells and cellular debris in the drug product solution.
Approach: We developed a multi-step approach to identifying and isolating the particulates from the drug product solution. After isolating the particulates, multiple techniques were employed to identify the particulates as being cellular or non-cellular in nature.
Result: Our team successfully isolated the unknown particulates and successfully identified the unknown particulates as both inherent particulates (cellular debris), and intrinsic particulates from the client’s manufacturing process.
CROs/CDMOs and Particulate Control
Engaging a team of expert scientists with combined decades of experience in particulate identification in biologic formulation can be the difference between a potential product recalls and ensuring a biologic formulation is safe and effective. Our team of consultative, knowledgeable scientists has proven success in identifying unknown particulates in biologics. They act as a true extension of your team, expanding your team’s knowledgebase and expertise. With a track record of successfully characterizing and understanding particulate load, source and composition of particulates in biologic formulations, they will work hand-in-hand with you to ensure the safety and efficacy of your biologic. Are you ready to talk specifics? Connect with an expert.
Find related Resources
Learn more

Biopharmaceuticals and Biologics Analysis
Element’s significant expertise in all stages of biologics R&D, from in-house protein biochemistry and molecular biology to cellular biology and QC experience, supports the entire product development life cycle.

Gene Therapy
Element uses a wide range of orthogonal analytical methods and capabilities to ensure the safety, identity, quality, purity and strength of gene therapies.

Therapeutic Protein Analysis
Element provides a variety of therapeutic protein analysis and characterization services to design molecule-specific analytical strategies in support of IND and BLA regulatory pathways.

Aseptic Manufacturing & Sterile Fill-Finish
Element offers comprehensive sterile fill-finish solutions tailor-made to support clinical studies, compounding pharmacies and small-scale batches, including small bulk fills.