Biomarkers 101: Intro to Biomarker Analysis
This article offers a thorough introduction to biomarker analysis, outlining the FDA's BEST Resource definitions and classifications. It examines the roles of biomarkers in the development of drugs and devices, discussing various categories such as safety, diagnostic, susceptibility/risk, prognostic, and predictive markers. Additionally, the article highlights the significance of validating biomarkers in clinical research.
The US Food and Drug Administration’s (FDA’s) Biomarkers, Endpoints, and other Tools (BEST) Resource is a biomarker and endpoint terminology and application glossary intended for basic medical research, medical product development and clinical care.
By harmonizing the terms used in translational science and medical product development, as well as focusing on terms associated with both biomarker analysis and clinical study endpoints, the BEST Resource expects to achieve its goals of improving communication, aligning expectations, and increasing scientific understanding. Several fundamental topics the BEST Resource addresses, including the definitions and categorizations of biomarkers (Figure 1), as well as the process of biomarker validation, will be discussed throughout this article.
Biomarkers defined
The term biomarker is an abbreviation of “biological marker.” Generally, biomarkers are indicators of biological processes within cells or organisms, as they are measurable substances. As stated by the BEST Resource, biomarkers are “used to measure normal biological processes, pathogenic processes or responses to an exposure or intervention.” Biomarkers play a critical role in the development of drugs and medical devices, producing valuable data throughout the entire drug development lifecycle.
Categories of biomarkers
Biomarker strategies can be categorized into the following groups:
- Safety
- Diagnostic
- Susceptibility/risk
- Prognostic
- Predictive
- Pharmacodynamic/response
- Monitoring
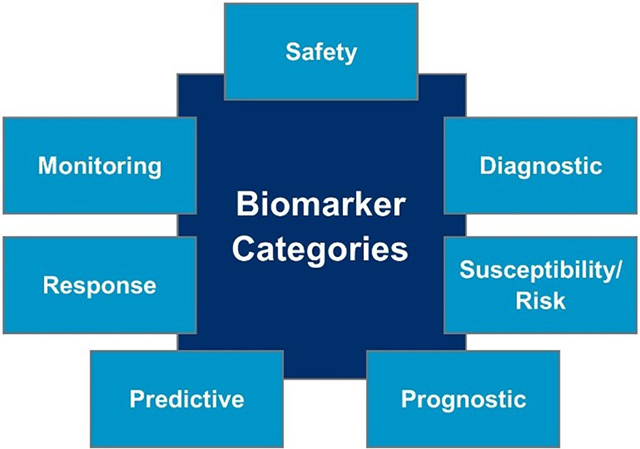
Figure 1. Biomarker categories
Safety biomarkers
Crucial safety assessments (e.g., hepatic panels), which rely on the use of safety biomarkers, are employed throughout drug discovery and development. Safety biomarkers are a specific marker of early clinical injury and are utilized to inform safety assessments throughout the process of drug development. It is essential to identify and qualify preclinical safety biomarkers with “fit-for-purpose” translational specificity and sensitivity for challenging targets, including those with novel disease indications and known narrow therapeutic indices.
Diagnostic biomarkers
The determination of the presence or confirmation of a particular disease or condition is aided with the use of diagnostic biomarkers. Although they are primarily used to establish whether a patient has a particular disease or condition, diagnostic biomarkers can also identify the specific disease subtype within a patient, such as HER-2 positive/negative breast cancer. In clinical settings, physicians rely on diagnostic biomarkers, in addition to physical examinations and empirical observations, to diagnose diseases promptly and accurately.
Susceptibility/risk biomarkers
Susceptibility/risk biomarkers are associated with an increased, or in certain cases, decreased, probability of developing a medical condition in individuals that have not yet presented that medical condition or disease. In clinical practice, susceptibility/risk biomarkers steer preventative strategies, in contrast to the use of susceptibility/risk biomarkers for clinical trial enrichment during medical product development. For instance, certain biomarkers may be monitored to enable the targeted use of vaccines in patients with a higher likelihood of developing a specific disease.
Prognostic biomarkers
Prognostic biomarkers are used to identify the increased or decreased likelihood of a future clinical event, disease recurrence, substantial worsening in condition, or disease progression in individuals with a particular medical condition or disease. Prognostic biomarkers are often used as an eligibility requirement in clinical trials, as they enable the identification of participants who are more likely to experience disease progression or clinical events throughout the course of a clinical trial.
Predictive biomarkers
Information about treatment benefits can be gleaned from predictive biomarkers, and these biomarkers can also be a target for therapy. Predictive biomarkers can be used to identify patients and individuals who are more likely to respond (e.g., symptomatic benefits, improved survival rates, adverse effects) when exposed to a specific medical product or environmental agent. Nevertheless, benefit is not guaranteed even if predictive biomarkers are identified and found to be present in an individual. Predictive biomarkers are often confused with prognostic biomarkers; to better understand the differences between these two types of biomarkers, see Figure 2.
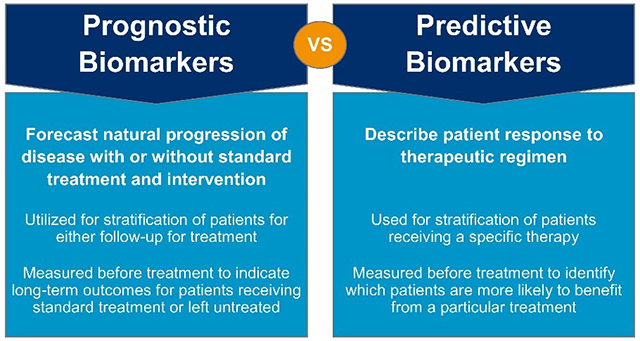
Figure 2. The differences between prognostic and predictive biomarkers
Pharmacodynamic/response biomarkers
Pharmacodynamic/response biomarker levels change in response to exposure to an environmental agent or medical product, and these biomarkers can be used to:
- Provide early evidence that a treatment may have an effect on a clinical endpoint of interest
- Assess a pharmacologic endpoint associated with safety concerns
- Provide useful information for patient management decisions
- Assess pharmacodynamic (PD) effects correlated to clinical effects during medical product development
The FDA’s BEST Resource states that pharmacodynamic/response biomarkers may be useful in establishing proof-of-concept that a specific drug is producing a pharmacologic response in humans believed to be related to clinical benefit, as well as to guide dose-response studies. Frequently, it is very challenging to statistically power an early-phase clinical trial to demonstrate meaningful change in a clinical outcome. Consequently, an extended period of time is needed before a meaningful change can be demonstrated for many clinical outcomes. In these instances, evidence of target engagement can be demonstrated with pharmacodynamic/response biomarkers.
Monitoring biomarkers
The BEST Resource describes monitoring biomarkers as biomarkers that are evaluated repeatedly over time, and can be used to assess:
- Disease progression, including the manifestation of new disease effects, worsening of previously occurring abnormalities, or shifts in disease severity or specific abnormalities, and/or
- The response of a disease or condition to treatment, either favorable or unfavorable.
Biomarker validation
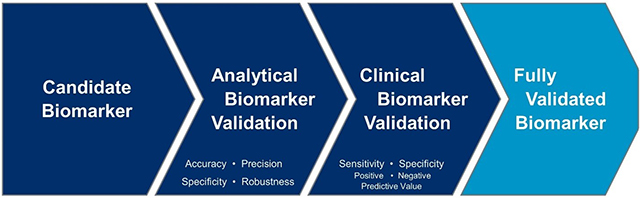
Figure 3. Biomarker validation progression pathway
When analyzing biomarkers, it is critical to establish that tests measure what was intended (i.e., analytical validation), as well as to make certain that the biomarker test can predict or measure relevant clinical concepts (i.e., clinical validation), as shown in Figure 3.
Analytical biomarker validation
Analytical biomarker validation ensures and establishes that when a specified protocol is followed, the performance characteristics of the biomarker assay or device are acceptable and meet established accuracy, specificity, precision, and other performance criteria.
Clinical biomarker validation
Clinical biomarker validation confirms that the test or device performs as intended and identifies, measures, or predicts the clinical, biological, physical, or functional state that it is intended to capture or exhibit.
Choosing the right bioanalytical partner for biomarker analysis
Selecting the right bioanalytical partner is just as critical as biomarker identification throughout the drug development lifecycle. Element’s team of consultative, expert scientists stand ready to support drug development from discovery through clinical trial and beyond, delivering high-quality results and biomarker analyses on your timeline. From bioanalytical method development, transfer and validation to cytotoxicity, clearance assays, drug metabolism and pharmacokinetics (DMPK) support, and therapeutic drug monitoring (TDM), our team offers comprehensive, integrated bioanalytical support for both routine bioanalysis and complex problem-solving.

