FAQ: What is Hydrogen Embrittlement?
We asked our metallurgy and failure analysis expert Vikram Nanda to answer the Frequently Asked Questions he and his team receive daily regarding hydrogen embrittlement. Read his answers below to discover the causes, effects, prevention of hydrogen embrittlement, and much more.
What is hydrogen embrittlement (HE)?
Hydrogen Embrittlement (HE) is characterized by a loss of ductility and strength due to the absorption of hydrogen atoms by the metal, which eventually diffuses along the grain boundaries, weakens the material structure, and promotes Intergranular Cracking. As the metal strength increases, steel becomes harder, less ductile, less tough and becomes more susceptible to HE. The result of hydrogen embrittlement is that steel components crack and fracture by brittle morphology at stresses less than the yield strength of the metal.
What is hydrogen embrittlement in steel/stainless steel?
Hydrogen embrittlement occurs most notably in high-strength steels, while stainless steels are known to be relatively less susceptible to hydrogen embrittlement but not totally immune to HE. Hydrogen embrittlement is essentially a loss of ductility and reduction of load-bearing capability of a relatively ductile metal due to the absorption of hydrogen atoms into the metal either during manufacturing, fabrication, or in service. Hydrogen atoms are small and can easily permeate solid metals.
Once absorbed, atomic hydrogen recombines in the various trapping sites like voids/inclusion sites or internal defects in the material to form hydrogen molecules (H2), which create pressure from within the metal. This pressure can increase to the levels where cracks initiate and eventually lowers the stress required for these cracks in the metal to propagate, resulting in embrittlement (brittle fracture).
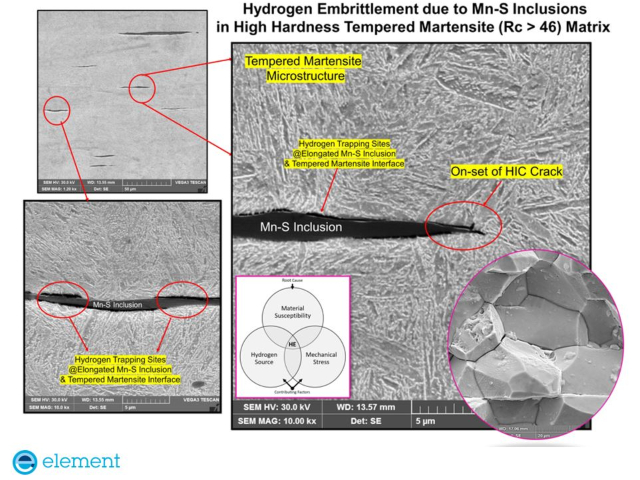
How does hydrogen embrittlement happen in bolts?
Hydrogen embrittlement (HE) in high-strength steel bolts of property classes 10.9 & 12.9 is known to happen primarily due to changes in material behavior by atomic hydrogen. As a result, this may lead to brittle fracture under tensile load caused by the tightening procedure or during in-service conditions.
What is hydrogen embrittlement temperature?
Hydrogen embrittlement (HE) is a near-ambient temperature phenomenon. HE gradually diminishes when the temperature is higher than 100°C.
What causes hydrogen embrittlement?
Hydrogen embrittlement is caused by the absorption of hydrogen atoms into a material having hydrogen trapping sites within the microstructure. This can lead to a loss of ductility and toughness under applied or residual stresses and promote cracking and brittle fracture at stress level which is less than the yield strength of the metal. The source of hydrogen atoms could be corrosion reactions, electroplating processes, acid pickling, or fabrication processes like welding by using damp welding rods. In general, high-strength materials are more susceptible to HE.
How to prevent hydrogen embrittlement in electroplated fasteners?
One of the most common procedures to prevent hydrogen embrittlement is through a process called baking. Baking normally consists of heating the electroplated fasteners to a specified temperature and holding them at a temperature generally between ~180 - ~220ºC for a period of 8 to 24 hours.
Which metal is the most vulnerable to hydrogen embrittlement?
Hydrogen embrittlement (HE) does not affect all metallic materials equally. The most vulnerable materials are high-strength steels (such as those used in the aerospace and automotive industries), as well as titanium and cobalt alloys whereas other materials such as copper, aluminum, stainless steels, and some nickel alloys, have relatively low susceptibility to HE. However, if these materials have gone through a strain-hardening process, then they may get affected by hydrogen embrittlement.
Chrome plating, zinc plating, and electroless nickel plating may also cause HE. It is important to note that the vulnerability of material to hydrogen embrittlement can be affected by a variety of factors, such as the presence of impurities in the material, the level of hydrogen exposure during manufacturing/fabrication or in service, stress level (applied and/or residual), and the temperature and pressure conditions in which it is being used.
How do you identify hydrogen embrittlement?
The most effective inspection methods to identify hydrogen embrittlement cracking are magnetic particle testing (MPT) and ultrasonic testing (UT). MPT is particularly useful for identifying surface cracks, however, from my experience, implementing MPT on small fasteners, could be a challenge.
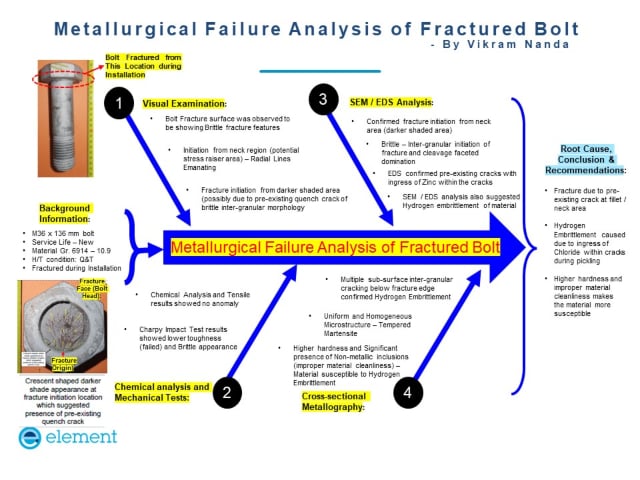
How can Element help?
Element laboratories in the UK, US, and Middle East can perform mechanical hydrogen embrittlement testing according to ASTM F519. The test specification outlines the use of Sustained Load Testing (SLT) to measure the possibility of hydrogen embrittlement in steel materials by applying uniaxial tension for up to 200 hours.
Contact us to learn more about how we perform hydrogen embrittlement testing.
Related Services

ASTM F519 Hydrogen Embrittlement (HE) Testing Services
Element's ASTM F519 hydrogen embrittlement testing evaluates material performance under tensile stress, identifying vulnerabilities in high-strength steels and alloys to prevent failures in aerospace, automotive, and energy applications.

Ultrasonic Testing (UT) Services
Detect hidden defects with Element's ultrasonic testing services. Our expert teams provide both contact and immersion UT inspection to verify component integrity, meet standards, and prevent failures.

Magnetic Particle Inspection (MPI) Services
Element's experts carry out non-destructive magnetic particle inspection tests to check for surface and near-surface discontinuities in ferrous metals.

Hydrogen Induced Cracking (HIC) Testing
Prevent critical equipment failure with our fast-turnaround Hydrogen Induced Cracking Testing. Expert NACE-compliant analysis ensures your oil & gas components are safe for sour service environments.


