Material Sciences Testing
Materials testing is crucial for the pharmaceutical industry. to understand the chemical composition of a substance, whether analyzing contaminants in a known substance or identifying the ingredients of an unknown substance. Through advanced testing equipment and collaboration with our network of material testing laboratories, Element offers pharmaceutical companies a complete suite of material testing services. We deliver the certainty you need for safety, quality, and performance.
Why Element are global leaders in Material Sciences Testing ?
Pharmaceutical companies face increasing pressure to ensure drug safety, meet stringent regulatory requirements, and accelerate time-to-market while maintaining quality standards. Material contamination, unknown substance identification, and compliance validation are critical challenges that can impact patient safety and regulatory approval timelines.
Element’s pharmaceutical material testing services support drug development, manufacturing quality control, and regulatory compliance processes. Our services can be used to aid in the development of chemical products, to analyze product failure cases in manufacturing, for particle or residue testing to identify substances, and much more.
Our extensive capabilities across multiple laboratories mean you get all your testing needs met through a single partner, streamlining your quality assurance process and saving valuable time. Our materials testing capabilities include material selection, performance, characterization and failure analysis.
Our cGMP-compliant laboratories and rapid turnaround times ensure your testing aligns with pharmaceutical production schedules and regulatory submission deadlines. We understand the science behind your materials and their performance and support you through all material testing procedures while ensuring your commercial requirements and production timescales are met.
Services

Chemical Characterization
Element's chemical characterization services help identify, analyze, and ensure the quality of materials, addressing challenges in purity, composition, and contaminants with advanced techniques and expertise.

X-Ray Diffraction (XRD) Analysis
X-Ray Diffraction (XRD) analysis is a non-destructive testing method that identifies crystalline phases and chemical composition, supporting precise material characterization for industries requiring detailed structural insights.

Physical Characterization of Pharmaceutical Products services
Our expert physical characterization scientists apply a variety of techniques, from SEM to TGA, to help you understand the physical properties of your pharmaceutical materials.

Solid-State Characterization of Active Pharmaceutical Ingredients (APIs) and Excipients
Element's solid-state characterization services streamline drug development, improving API performance, stability, and manufacturability. Our tailored strategies and expert teams address key pharmaceutical challenges for faster results.

Contaminant Analysis and Complaints Investigations
Our contamination detection and analysis services isolate and identify unknown particulates and contaminants in pharmaceuticals and biologics down to trace levels.

Particle Size Distribution (PSD) Testing and Analysis
Element's experts provide specialist particle analysis services for Particle Size Distribution (PSD) and particle counting following USP, EP, ASTM and ISO methods.

Rubber, Polymer, & Plastic Testing
Element offers tailored rubber, polymer, and plastic testing services, supporting quality control, failure analysis, and product development with advanced techniques and expert insights for pharmaceuticals.

Pharmaceutical XRD Testing
Element's pharmaceutical XRD testing delivers precise crystalline structure analysis with minimal sample requirements. Our advanced equipment and 30+ years of expertise help you meet regulatory requirements faster. Contact our experts today.
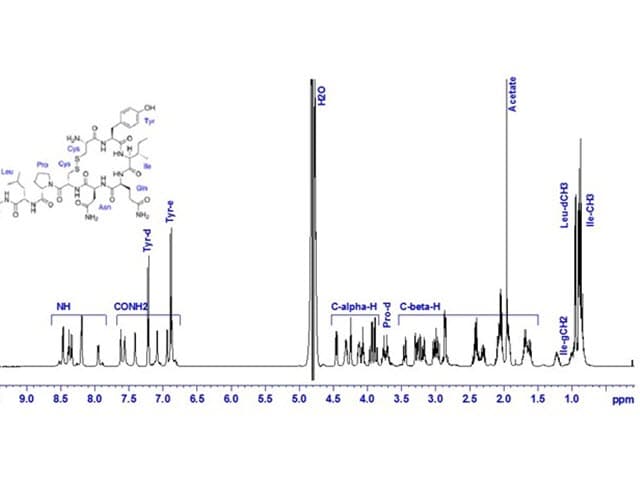
Nuclear Magnetic Resonance (NMR) Spectroscopy in Pharmaceutical Analysis
Element's Nuclear Magnetic Resonance (NMR) Spectroscopy analysis supports critical stages of pharmaceutical development, enabling verification of chemical synthesis and compound characterization.
Your Challenges, Our Solutions
Material Composition Certainty
Product Failure Investigation
Meeting Production Deadlines
Standards we test to and materials we test
We can conduct analytical testing and material analysis in accordance with USP, EP, ASTM standards and cGMP compliance.
Thanks to our expert staff and advanced laboratory capabilities, we can identify a material’s chemical composition with an incredible degree of precision, analyzing compounds for:
- Active Ingredients
- Contaminants
- Fillers
- Particulates
- Solvents
- Plastics
- Polymers
- Additives
- Unknown Materials
- Excipients
- Raw Materials
- Drug Product Components
Why Choose Element


