Nickel Ion Release Testing
Ensure your nitinol medical devices meet safety and regulatory requirements with comprehensive nickel ion release testing. Our cutting-edge dynamic testing methods combine fatigue analysis with precise ion release measurement, helping you validate both initial and long-term nickel leaching under real physiological conditions. Get the data you need for FDA compliance while protecting patient safety.
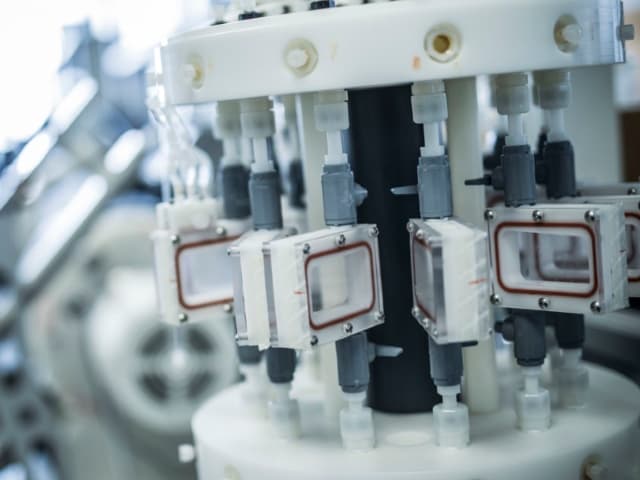
What is Nickel Ion Release Testing at Element?
Nickel ion release testing quantifies the amount of nickel that leaches from medical devices into physiological fluids over time. At Element, we specialize in evaluating nickel release from nitinol and other nickel-rich medical devices, particularly those used in minimally invasive procedures, using both static and dynamic testing approaches to ensure comprehensive assessment.

What can Element offer you for Nickel Ion Release Testing?
Products We Test
Products We Test
Your nitinol and nickel-containing medical devices require thorough evaluation to ensure patient safety. At Element, we test a comprehensive range of devices, from intravascular stents to minimally invasive implants, providing detailed analysis of nickel release patterns under various conditions.
Key Tests Offered
Key Tests Offered
- Initial bolus release quantification
- Long-term nickel ion release profiling
- Combined fatigue and ion release testing
- Dynamic pulsatile testing with ion capture
- Physiological condition simulation
Methods And Solutions Offered
Methods And Solutions Offered
Our innovative testing approach combines mechanical fatigue testing with continuous nickel ion release measurement. This custom-developed methodology allows for real-time capture of released ions during dynamic loading, providing more accurate data than traditional static testing alone.
Nickel release testing measures the amount of nickel leached from a device into a fluid at physiologic temperature and pH. For permanent implants, testing typically lasts at least 60 days.
During fatigue testing, nickel ion release is closely monitored under conditions that mimic the body. This captures both the initial burst and long-term release of nickel ions in vitro.
As the device undergoes radial fatigue, a small volume of test media absorbs the released nickel ions. The media is extracted at multiple time points, with extractions focused on the early stages to capture the initial release. The amount of released nickel is measured using inductively coupled plasma mass spectrometry (ICP-MS).
Cutting-Edge Equipment We Use
Cutting-Edge Equipment We Use
- Custom test setups for dynamic testing
- Inductively coupled plasma mass spectrometry (ICP-MS)
- Low volume reservoir systems
- Physiological temperature and pH control systems
Which Labs Offer This Service
Which Labs Offer This Service
Our team operates from life science hubs across the world, providing global access to our expert capabilities. Find your nearest life science testing hub on our Locations Page.
Proven Research Partnership
Products we test
- Nitinol-based medical devices
- Intravascular stents
- Shape memory implants
- Minimally invasive devices
- Other nickel-rich medical devices
- Cardiovascular implants
- Intravascular devices
Your Challenges, Our Solutions
Patient Safety Concerns
Regulatory Compliance Requirements
Real-World Performance Data
Standardization Challenges
Why Choose Element

Industry-Leading Methodology
Comprehensive Assessment
Dynamic Testing Innovation
Flexible Solutions
190+years of testing expertise
60+days of testing capability
8,500+engaged experts
10+years simulation capability

Related services

Medical Device Testing Services
With Element as your medical device testing partner, you’ll enjoy the benefit of a single comprehensive supplier across feasibility, R&D and prototype trials, through product development, regulatory validation and production quality control.

Coating & Particulate Testing
Element provides coating integrity and particulate testing for intravascular devices, helping manufacturers meet safety standards, improve device performance, and comply with regulatory guidelines.

Stent & Stent Graft Testing Services
Element provides expert stent testing services to validate cardiovascular device compliance, durability, and performance. Our comprehensive testing meets regulatory standards, supporting safe and effective device
Frequently asked questions
How long does nickel ion release testing take?
For permanent implants, we recommend testing for at least 60 days to capture both initial bolus release and long-term leaching patterns.
Why is dynamic testing important for nickel release?
Dynamic pulsatile fatigue loading accelerates nickel release compared to static conditions, providing more accurate real-world performance data.
How does your nickel ion release testing process work?
We capture nickel ions in a specialized low-volume reservoir while your device undergoes radial fatigue testing. The test media is extracted at strategic intervals, with more frequent sampling early on to capture initial release patterns. We then use ICP-MS to precisely quantify the nickel content.
