Coating & Particulate Testing
Our coating and particulate testing services help validate intravascular devices for patient safety and therapeutic benefit. We provide acute and chronic particulate testing, coating integrity assessment, and durability testing aligned with FDA guidance requirements.

What is Medical Device Coating & Particulate Testing at Element?
Coating and particulate testing evaluates coating integrity and measures particulate matter released during device use. At Element, we examine coatings and particulates for intravascular devices according to industry standards and FDA guidance documents.

What Can Element Offer You For Coating & Particulate Testing of Medical Devices?
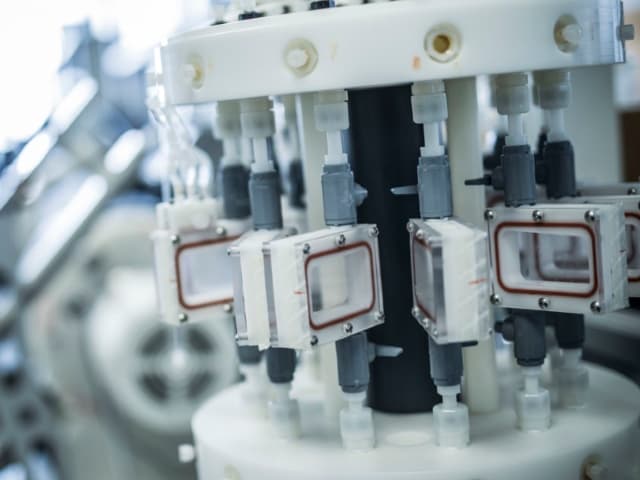
Components and products we test
Components and products we test
Element tests intravascular devices including drug-eluting stents, examining coated surfaces for defects, anomalies, or artifacts. Our testing covers both metallic and polymeric materials used in cardiovascular devices. We evaluate drug-eluting stents for coating integrity and durability, focusing on potential impacts to therapeutic benefit and clinical performance.
Key tests offered
Key tests offered
We provide acute and chronic particulate testing, coating integrity inspections for baseline and simulated use conditions, and particulate evaluation through light obscuration or filtration/microscopy methods. Our testing includes counting total defects per unit area, measuring representative and worst-case defect areas, and conducting spectral analysis and element mapping.
Methods and solutions offered
Methods and solutions offered
We employ filtration/microscopy methods for detailed particle identification and quantification. Our simulated use testing includes tortuous path fixtures that mimic challenging in vivo conditions. We conduct testing on finished products that have undergone all manufacturing processes, including sterilization, and can evaluate overlapped or bent stent configurations following device instructions for use.
- Coating Integrity Inspections:
Coating integrity inspections assess the device's coated surfaces for defects, anomalies, or damage that could impact clinical performance. Issues like delamination, flaking, or degradation must be identified and characterized. FDA guidelines recommend inspections of the finished product after all manufacturing processes, including sterilization.
Simulated Use Testing
We perform coating integrity assessments under both baseline and simulated use conditions. Devices are subjected to a tortuous path fixture that simulates challenging in vivo conditions and then expanded in air or an aqueous solution.
Defect Documentation and Reporting
Defects are carefully documented, including their size and quantity, as part of the coating integrity evaluation. Test reports include details on how defects were quantified, such as counting defects per unit area and measuring representative and worst-case defect sizes.
- Acute Particulate Testing:
FDA guidance and industry standards outline acute particulate testing, using either light obscuration or filtration/microscopy methods. The light obscuration method offers high throughput but only categorizes particulates by size. On the other hand, the filtration/microscopy method can quantify and identify particulates by size, color, shape, or composition. We use the filtration/microscopy method for its flexibility in meeting device manufacturers' specific needs.
- Chronic Particulate Testing:
Our chronic particulate testing integrates particle counting modules with stent graft test systems and uses customized flow loops, flow meters, and filters for mock vessels. This setup minimizes flow compliance effects, ensuring accurate testing under physiological conditions.
Filters are sampled at specific intervals during long-term fatigue tests to analyze particulates. With our in-house filtration/microscopy capabilities, we can quickly analyze particulates while the durability test is ongoing.
Cutting-edge equipment we use
Cutting-edge equipment we use
We utilize SEM with automated particle size analysis, providing magnification up to 100,000x. Our sample chamber accommodates samples up to 100mm long x 80mm wide x 25mm high. The system includes Automatic Feature Analysis (AFA) capabilities for spectral analysis, line scans, and element mapping of solid inorganic materials.
Which labs offer this service
Which labs offer this service
Our team operates from strategically located Life Sciences labs, providing convenient access to our expert capabilities. Find your nearest Life Sciences hub on our Locations Page.
Integrated durability testing
Integrated durability testing
We combine particle counting modules with stent graft test systems and individualized flow loops. Our design minimizes flow compliance effects while maintaining physiological radial strains for fatigue testing. Filters for mock vessels are sampled at pre-determined intervals throughout long-term fatigue tests.
Standards we test to and components we test
Several standards support coating inspections and particulate testing:
- ASTM F2743 Standard Guide for Coating Inspection and Acute Particulate Characterization of Coated Drug-Eluting Vascular Stent Systems
- AAMI TIR42 Evaluation of particulates associated with vascular medical devices
- USP 788 Particulate Matter in Injections
- ASTM F2477 Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
- ISO 25539-1 Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
- ISO 25539-2 Cardiovascular implants – Endovascular devices – Part 2: Vascular stents
- Drug-eluting stents
- Intravascular devices
- Cardiovascular guidewires
- Vascular stent systems
- Endovascular prostheses
Your Challenges, Our Solutions
Clear particulate characterization
Replicating challenging physiologic conditions
Durability performance validation
Fast analysis turnaround
Why Choose Element

Comprehensive testing solutions
Advanced analytical capabilities
Customized test protocols
Integrated monitoring systems

Explore our global network of labs and find your nearest location
VIEW ALL LOCATIONSRelated services

Medical Device Testing Services
With Element as your medical device testing partner, you’ll enjoy the benefit of a single comprehensive supplier across feasibility, R&D and prototype trials, through product development, regulatory validation and production quality control.

Cardiac & Endovascular Device Testing
Element offers comprehensive cardiac and endovascular device testing, providing fatigue and durability analysis to support regulatory compliance and product reliability for stents, heart valves, pacemaker leads, and more.
Nickel Ion Release Testing
Element evaluates nickel icon release from nitinol and other nickel-rich medical devices to help medical device manufacturers characterize nickel ion leaching.

ISO 10555 Catheter Testing services
We offer comprehensive testing services for catheters and balloons under ISO 10555 standards for testing intravascular sterile use catheters.
Silicone Mock Vessels
Our cardiovascular device testing experts have extensive experience designing custom mock vessels for pulsatile fatigue testing and other test methods for devices such as stents, occluders, and heart valves.

Stent & Stent Graft Testing Services
Element provides expert stent testing services to validate cardiovascular device compliance, durability, and performance. Our comprehensive testing meets regulatory standards, supporting safe and effective device
