
Ask a Question or Request a Quote
Talk to an expert today
Bringing innovative medical device technology to the marketplace is a high‐risk venture. That’s why Element strives to provide absolute testing certainty by delivering accurate and reliable results for our clients’ medical device testing needs, from small, start-up ventures to large, established manufacturers.
Our full product life cycle approach to medical device testing
Element supports every stage of medical device testing - from test protocol development and prototype/feasibility trials to testing and consultation for:
Our turnkey invasive and non-invasive Physiological Monitoring Studies cover the entire product lifecycle, from R&D to validation for regulatory submission.
Medical device testing methods with Element
We offer a full suite of medical device testing, including:
- Mechanical Testing
- Product Testing
- Clinical Validation Testing
- Wireless Device Testing
- Material Characterization
- Microbiological Evaluation
- Biocompatibility & Toxicology Testing
As a comprehensive medical device testing partner, you’ll enjoy the benefit of a single source supplier for all your testing needs, from feasibility and R&D to product development and production quality control.
Medical device testing solutions that you can trust
Our dedicated team of experts have years of experience in a wide range of testing services for Class, I, II and III medical devices to help you meet regulations and ensure that every aspect of your medical device product is properly tested.
Contact us today to learn how Element can provide testing services and consultation for your medical device.
Our Medical Device Services
Element offers the most comprehensive range of materials and product testing services for the medical device industry.

Biocompatibility and Toxicology Testing Services
Element's biocompatibility and toxicology services provide fast, reliable, and compliant testing solutions that accelerate your medical device development while ensuring the highest standards of safety and regulatory compliance.

Orthopedic Implant Testing
As a global leader in orthopedic implant testing, Element has years of experience in evaluating hip replacements, knee prostheses, spinal devices and many other implants.
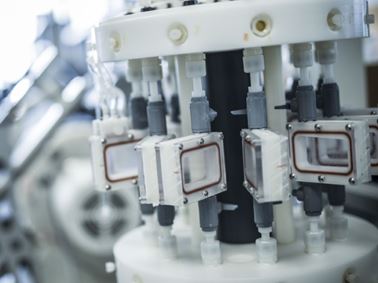
Cardiovascular Device Testing
Element specializes in a wide range of testing for cardiovascular devices, such as stents, grafts, occluders, catheters, heart valves and pacemaker leads.

Product Specific Regulatory Compliance RoadMap
RegNav combines expert insights with AI software, giving you a detailed picture of your medical device’s safety and performance requirements.
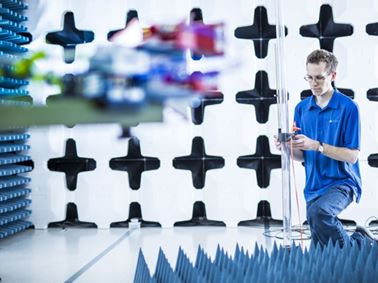
EMI & EMC Testing
Element performs electromagnetic compatibility (EMC) and electromagnetic interference (EMI) testing for a variety of medical devices and components, including implantable devices, diagnostic tools and therapeutic equipment.

Reusable Medical Device Reprocessing Validation and Testing
Every Reusable Medical Device must be reprocessed before reuse to minimize risk of contamination; this includes cleaning and either sterilization or disinfection.

Reusable Medical Device Life Cycle Validation
Our medical device experts evaluate the environmental impact on the identification markings and function of reusable medical instruments after repeated autoclave sterilization cycles.

Chemical Compatibility Testing for Medical Devices, Materials, and Surfaces
Element can offer automated chemical compatibility testing that gives faster and more reliable results for medical device compatibility with medical disinfectants.
Extractables and Leachables Studies
Element's extractables and leachables studies offer tailored solutions that ensure patient safety and compliance with industry standards.
Microbiological Analysis
Element provides a wide range of microbiological analysis projects for medical devices, from pathogen and bioburden testing to endotoxin and cytotoxicity analyses.

Rated Burst Pressure & Leak Testing
Element specializes in performing rated burst pressure and leak testing for medical devices, including catheters, balloons, valves, medical bags, tubing, and connectors.

Accelerated Aging Shelf Life Testing
Our shelf life testing can help you determine how climatic and environmental factors like humidity, temperature, and light will affect the lifespan and integrity of your products.

Medical Device Battery Testing
Element provides safety and certification testing for rechargeable lithium-ion and nickel metal hydride batteries used in hospital and home health applications.

IEC 60601 Safety Testing
Element provides provide a full suite of medical device testing and certification services to ensure that you meet the necessary IEC 60601 product safety requirements recognized across the world.

Medical Device Regulatory Services
Our medical device regulatory experts help manufacturers of medical devices with product registrations, quality management systems, and in-country and global approvals.

Clinical Validation Testing
Focus on growing your business with Element’s turn-key invasive and non-invasive physiological monitoring studies of medical devices and wearables.

Syringe Testing
With extensive experience evaluating prefilled syringes and autoinjector components for functionality and integrity, Element can help you successfully navigate regulatory requirements for testing.

Analytical Testing Services
We offer a comprehensive array of analytical testing services and expert scientific consultancy from our GMP/GLP and ISO/IEC 17025 accredited laboratories, to meet the needs of clients across the life sciences sector.
Our Medical Device Testing Services

Life Sciences Content
Check out our Life Sciences thought leadership content including webinars, white papers, case studies blogs and more.
Written by our industry experts, we tackle a range of subjects across Antimicrobial, Biotechnology Food, Medical Device, Pharmaceutical, and Personal Care industries.
With a commitment to absolute certainty, we utilize our extensive expertise and experience to provide insights that ensure the safety of materials used in the life science sector.
READ MORE