Clinimark is part of Element

 We're thrilled Clinimark is part of Element. We continue to provide the same high quality clinical validation services and expertise you've come to expect from us. Plus, you now have access to a greater depth and breadth of testing, certification, and advisory services. Together, we'll bring optimized devices to market, leveraging Element's global platform and world-leading experts.
We're thrilled Clinimark is part of Element. We continue to provide the same high quality clinical validation services and expertise you've come to expect from us. Plus, you now have access to a greater depth and breadth of testing, certification, and advisory services. Together, we'll bring optimized devices to market, leveraging Element's global platform and world-leading experts.
Save time and money with a single supplier
Save time and money when you partner with Element from prototype to market. Our global, integrated services platform has been built to cut costs and reduce time to market for medical devices and wearables. Interested in learning more? We still offer the same core clinical validation testing services you've come to expect from us - look below for more. Keep scrolling to explore a handful of expanded services we now offer as part of Element. To know more about Element, scroll further down to learn about our purpose, values, history, and more.
Find our core services
For more information about the core services we continue to provide as Element, click the links below.

Clinical Validation Testing
Element delivers comprehensive clinical validation testing for medical devices and wearables, managing study design, recruitment, testing, and data analysis. Our complete solutions help overcome regulatory challenges and accelerate product development.
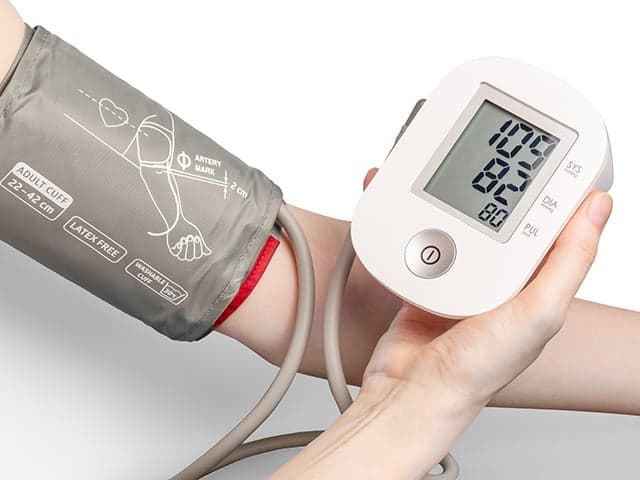
Blood Pressure Monitor Clinical Validation Testing
Element provides comprehensive blood pressure monitor clinical validation testing—from protocol design and participant recruitment to data analysis—leveraging invasive and non-invasive methods to overcome regulatory challenges and deliver precise device performance.

Heart Rate Monitor Device Validation
Element provides clinical validation testing for heart rate monitors using human and simulator data, meeting regulatory standards while delivering accurate performance. Their expert guidance addresses key accuracy challenges.

Pulse Oximeter Validation Testing
Element's pulse oximeter clinical validation service provides comprehensive human testing that delivers real-world, high-quality data to support regulatory submissions and significantly optimize device performance across diverse clinical settings with precision.
Respiratory Rate Monitor Clinical Validation Testing
Element offers expert-led respiratory rate monitor clinical validation testing, covering protocol design, participant recruitment, and regulatory-ready reports for streamlined submissions.

Sleep Monitoring Device Validation
Element provides clinical validation testing for sleep monitoring devices, supporting regulatory submissions and optimising device safety through expert-led data analysis and participant studies.

Temperature Monitoring Device Clinical Validation Testing
Element offers expert clinical validation testing for temperature monitoring devices, supporting regulatory requirements and fast-tracking your product to market with comprehensive study management.

Human Factors and Usability Testing
Element's human factors and usability testing simulates real-world device use to reveal user errors and optimize performance. The service supports FDA guidelines and enhances overall device safety and effectiveness.
Click on the orange "Get more information" button and fill out the form. A member of our team will be in touch with you.
Let us know what you're looking for
Explore expanded testing, certification, and advisory services
As part of Element, we offer a greater depth and breadth of services. Click the links below to explore our global service offerings.

Battery Safety Testing for Medical Devices
Battery safety testing for medical devices, assessing performance, reliability, and compliance with international standards to support safe use in healthcare settings.

Medical Device Regulatory Services
Element offers expert medical device regulatory consulting, guiding you through design, risk analysis, and FDA submissions for efficient product approval.

Wireless Coexistence Testing
Ensure your devices perform flawlessly in crowded environments with our comprehensive wireless coexistence testing. Achieve compliance, minimize interference risks, and access 161+ global markets—all with one trusted partner.

Medical Device EMC Testing
Element provides EMC testing and certification for Class I-III medical devices, helping manufacturers meet global regulatory standards and accelerate market entry with expert guidance and accredited laboratories.

Electronic Product Certification and Approvals Services
Accelerate your electronic product certification with Element's ISO 17065-accredited services. Access 167 markets through one trusted partner. Expert testing & compliance support.

Chemical Compatibility Testing for Medical Devices, Materials, and Surfaces
Our automated chemical compatibility testing verifies that medical devices withstand corrosive disinfectants, offering comprehensive, fast, and reliable evaluations that preserve product integrity and extend device longevity in harsh cleaning environments.

CE Marking Services
Accelerate EU market access with Element's CE Marking services. Expert testing, documentation & compliance support for electrical products. Get certified faster.

Electrochemical Corrosion Testing
Element offers electrochemical corrosion testing to identify material risks, enhance durability, and support informed material selection across industries.

Polymer Testing & Characterization Services
Maximize your polymer material performance with Element's comprehensive testing services. Our expert analysis translates complex data into actionable insights across your entire product lifecycle.

Wear Testing of Medical Devices
Element can handle every aspect of your medical device wear test program, no matter how long you want the tests to run for or how many repeats you need.
- Battery Safety Testing for Medical Devices
- Medical Device Regulatory Services
- Wireless Coexistence Testing
- Medical Device EMC Testing
- Electronic Product Certification and Approvals Services
- Chemical Compatibility Testing for Medical Devices, Materials, and Surfaces
- CE Marking Services
- Electrochemical Corrosion Testing
- Polymer Testing & Characterization Services
- Wear Testing of Medical Devices
Get to know Element, your global partner from prototype to market
Click the links below to learn more about Element's global organization.

About Element
Element is a leading global provider of Testing, Inspection, and Certification (TIC) services on a wide range of products, materials, processes, and services.

Our Purpose
Find out why Element does what it does, each and every day across all of the end-markets we serve
Our Values
Find out about the values that everyone at Element strives to live by consistently, each and every day.

Element leads TIC industry in commitment to net zero following top ESG rating
Element sets out its new, industry leading environmental ambition, committing to science-based targets and net zero emissions across its entire global business by 2035.

Local laboratories, global platform
Doing business with one of our laboratories enables our clients' access to our global platform of expertise, capacity and capabilities.

Element Events
Take a look at Element's up-coming industry events. Engage with our experts and learn more about Element's capabilities.

Element bolsters connected technologies and life sciences businesses with Clinimark acquisition
Clinimark is global leader for clinical FDA validation testing of vital sign data for medical and home health devices, and consumer wearable products.
Our team of over 9,000 Engaged Experts in North America, Europe, The Middle East, Australia, Asia and Africa are ready to help you.

